SECuRE trial advances: No dose limiting toxicities and strong preliminary efficacy data in first multi-dose cohort
2024-09-13 Clarity Pharmaceuticals HaiPress

Highlights
Cohort 4 of the SECuRE trial is the first to assess multiple cycles of 67Cu-SAR-bisPSMA at the highest dose of 12GBq.
The Safety Review Committee (SRC) assessed early data from the first 3 participants in cohort 4 who received 2 doses of 67Cu-SAR-bisPSMA. Two of these participants had completed the dose limiting toxicity (DLT) period and 1 will complete the DLT period by the end of September. No DLTs were observed to date,in line with cohorts 1,2 and 3.
The safety profile of multiple doses of 67Cu-SAR-bisPSMA remains positive,with almost all adverse events (AEs) being mild or moderate. Furthermore,almost all AEs have either resolved or improved at the last assessment.
Preliminary efficacy assessment from cohort 4 after 2 therapy cycles in 2 participants following completion of the DLT period showed that both participants exhibited greater than 60% drops in prostate-specific antigen (PSA) levels in weeks following their second dose.
The SRC recommended,based on this early data,the SECuRE trial proceeds to enroll the last 3 participants of cohort 4,after which Phase II of the study will commence (cohort expansion phase,with 14 patients),pending safety evaluation.
Clarity will host a webcast and conference call for shareholders on Wednesday 18 September,details below.
SYDNEY,Sept. 12,2024 -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"),a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer,is pleased to announce an update on the safety review of the first 3 participants enrolled in cohort 4 of the SECuRE trial who received 2 doses of 67Cu-SAR-bisPSMA. Cohort 4 is the final cohort in the dose escalation phase of the study,with participants receiving a minimum of 2 and a maximum of 4 doses of 67Cu-SAR-bisPSMA at 12GBq.
The SECuRE trial (NCT04868604)[1] is a Phase I/IIa theranostic trial for identification and treatment of participants with prostate-specific membrane antigen (PSMA)-expressing metastatic castrate-resistant prostate cancer (mCRPC) using 64Cu/67Cu-SAR-bisPSMA. 64Cu-SAR-bisPSMA is used to visualise PSMA-expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre,single arm,dose escalation study with a cohort expansion involving approximately 44 participants in the US and Australia. The overall aim of the trial is to determine the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of prostate cancer.
Cohort 4 is the first to explore the safety and anti-cancer effects of multiple therapy cycles of 67Cu-SAR-bisPSMA at the highest dose of 12GBq. This is the last cohort of the dose escalation phase,before the trial advances into its Phase II stage (cohort expansion) in 14 participants,pending safety evaluation.
Cohort 4 is designed as a "3+3" cohort,where participants will receive a minimum of 2 therapy cycles. Based on the positive safety profile observed in the first 3 cohorts of the SECuRE trial,a change to the dosing schedule of cohort 4 from "2 doses" to "up to 4 doses" has previously been approved by the SRC. This will allow participants who are benefiting from 67Cu-SAR-bisPSMA to receive 2 additional doses under the SECuRE trial (up to 4 doses in total) (Figure 1).
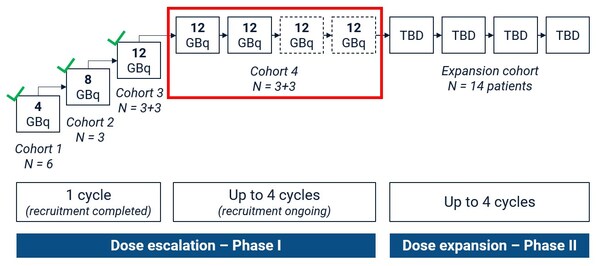
Figure 1. SECuRE Study Design. Participants in cohort 4 will receive a minimum of 2 doses and maximum of 4 doses of 67Cu-SAR-bisPSMA (12GBq) if no radiographic progression is observed. TBD: dose to be determined based on the safety assessment of cohort 4.
Safety & Efficacy
The SRC has reviewed the safety data of the first 3 participants of cohort 4 who have received 2 cycles of 67Cu-SAR-bisPSMA,and no DLTs have been observed to date. Two participants have completed the DLT period,and 1 participant will complete the DLT period by the end of September. Almost all AEs were mild to moderate,with the majority having resolved or improved at the last assessment. In the final participant who is yet to complete the DLT period,the only AE reported to date was nausea,which has resolved.
Early preliminary efficacy assessment shows a reduction in PSA levels following treatment in both participants who have completed the DLT period. In the weeks following the last therapy dose,these participants have already exhibited PSA drops of more than 60%. The largest drop in PSA to date was a fall of 92.3% (from a baseline PSA of 157.4 ng/mL),and it continues to decline based on the latest assessment. This participant,who had failed several lines of therapy prior to receiving 67Cu-SAR-bisPSMA (i.e. androgen deprivation therapy [ADT],androgen receptor pathway inhibitor [ARPI] and an investigational agent through a clinical trial),has already had a radiographic partial response based on Response Evaluation Criteria in Solid Tumours v1.1 (RECIST) assessment,with a reduction of 60.6% in tumour volume evaluated by PSMA positron emission tomography (PET) imaging thus far (Figure 2).
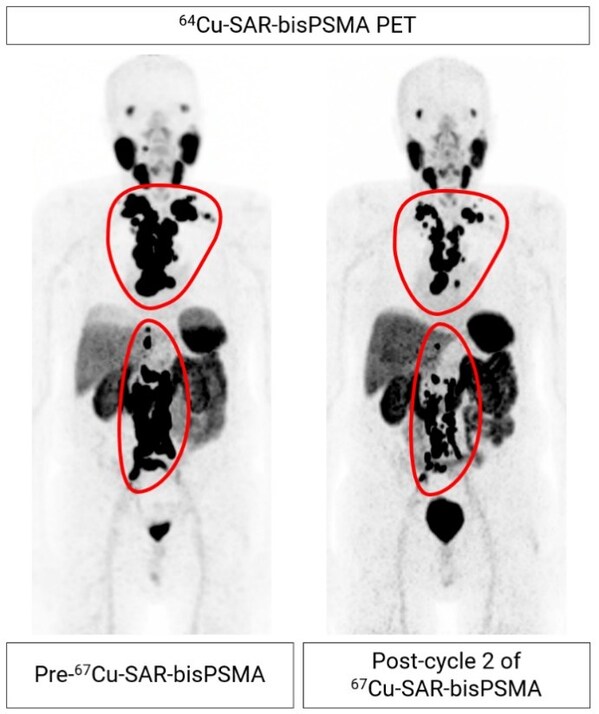
Figure 2. mCRPC patient from cohort 4 showing extensive metastasis of prostate cancer to the lymph nodes (regions highlighted by the red lines). 64Cu-SAR-bisPSMA images show reduction in tumour volume of 60.6% from pre- to post-treatment after two therapy cycles of 12GBq 67Cu-SAR-bisPSMA to date. PSA reduction of 92.3% (from 157.4 to 12.1 ng/mL) to date. Post-cycle 2 scan (64Cu-SAR-bisPSMA) performed approximately 8 weeks after the second dose of 67Cu-SAR-bisPSMA.
Clarity's Executive Chairperson,Dr Alan Taylor,commented,"The results from the SECuRE trial continue to impress,with the data from all 3 participants in cohort 4 exhibiting an excellent safety profile for 67Cu-SAR-bisPSMA,following 2 cycles of the product at the highest dose of 12GBq. The early approval by the SRC to progress with the trial and the excellent safety data to date reinforce our confidence that 67Cu-SAR-bisPSMA has a favourable safety profile as we consider the dosing schedule for the next phase of the trial. Although safety is our priority during this dose escalation phase,the efficacy data continues to be outstanding,with both participants who have thus far completed the DLT period analysis exhibiting an excellent response to treatment even at these early time points,and as shown in the image,in large amounts of bulky disease. It is important to note that participants who exhibited strong efficacy data in previous cohorts experienced the maximum effect several months after receiving 67Cu-SAR-bisPSMA (in some cases,almost 5 months after receiving one single dose of the product). Participants in this cohort,following completion of the DLT period,may now have the opportunity to receive additional doses of 67Cu-SAR-bisPSMA pending safety and efficacy assessments according to the clinical trial protocol,as we look to safely reduce the tumour burden or ultimately eliminate any remaining lesions in the body. The data from all cohorts is now presenting a unique proposition of a great safety profile coupled with remarkable efficacy,even in patients who had failed so many previous lines of therapy.
"We are now using this data to inform the next stages of our clinical development program,including the expanded cohort in the SECuRE study and subsequently the Phase III clinical trial prior to regulatory approval and full commercial rollout. The positive safety profile of 67Cu-SAR-bisPSMA,particularly compared to agents currently used to treat prostate cancer,lends itself to the treatment of patients much earlier in their disease,and we have commenced planning our clinical trials to focus on these earlier stages of prostate cancer.
"The treatment of early-stage disease is a sound approach to commercialisation for a therapy; however,given the outstanding efficacy data observed to date in late-stage patients with high PSA levels and who have failed multiple lines of therapies,this patient population is a priority for Clarity and clinicians. We are working closely with our Key Opinion Leaders and Clinical Advisers on strategies to address this high unmet need and continue to treat these patients whilst also progressing towards much earlier stages of prostate cancer. This is an incredibly exciting period for our Company,especially given the speed with which we have translated this proprietary product from the benchtop of Australian science only 5 years ago,to changing the lives of patients today. The high paced recruitment of this clinical trial,including the early commencement of recruitment for the final participants in cohort 4,coupled with our investigation of strategies to fast track the development and availability of this product to those patients who need it most,reflect our ambitions to better treat people with cancer."
Webcast and Conference Call
With a number of Clarity's recent developments,including Board and Executive advancements,inclusion into Australian Securities Exchange (ASX) indices,the changing mix of Shareholders on the Company's register as a result of this,and recent clinical and pre-clinical developments,Executive Chairperson,will host a webcast and conference call for analysts and Shareholders on Wednesday 18 September at 10.30amAEST.
Register via the links below:
Conference call: https://s1.c-conf.com/diamondpass/10041965-lk7a32.html
Webcast: https://webcast.openbriefing.com/cu6-mu-2024/
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis",which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure,called a chelator. Unlike other commercially available chelators,the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a TCT that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA are unregistered products. Individual results may not represent the overall safety and efficacy of the products. The data outlined in this announcement has not been assessed by health authorities such as the US Food and Drug Administration (FDA). A clinical development program is currently underway to assess the efficacy and safety of these products. There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death in men worldwide[2]. Prostate cancer is the second-leading causes of cancer death in American men. The American Cancer Institute estimates in 2024 there will be 299,310 new cases of prostate cancer in the US and around 35,250 deaths from the disease[3]..
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals,developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
ClinicalTrials.gov Identifier: NCT04868604,https://clinicaltrials.gov/ct2/show/NCT04868604
Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21834
American Cancer Society: Key Statistics for Prostate Cancer,https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
For more information,please contact:
Clarity Pharmaceuticals
Dr Alan Taylor
Catherine Strong
Executive Chairman
Investor/Media Relations
ataylor@claritypharm.com
catherine.strong@sodali.com
+61 406 759 268
This announcement has been authorised for release by the Executive Chairperson.
